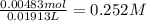Chemistry, 07.03.2020 19:41 Donlito8070
A 10.5 mL sample of vinegar, containing acetic acid, was titrated using 0.460 M NaOH solution. The titration required 19.13 mL of the base. What was the molar concentration of acetic acid in the vinegar?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

You know the right answer?
A 10.5 mL sample of vinegar, containing acetic acid, was titrated using 0.460 M NaOH solution. The t...
Questions

Physics, 08.07.2019 16:00


English, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00


Mathematics, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00



Physics, 08.07.2019 16:00

History, 08.07.2019 16:00

History, 08.07.2019 16:00

Mathematics, 08.07.2019 16:00



Mathematics, 08.07.2019 16:00