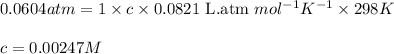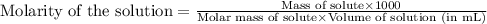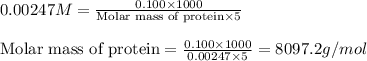Of an unknown protein are dissolved in enough solvent to make 5.00mL of solution. The osmotic pressure of this solution is measured to be 0.0604atm at 25.0°C. Calculate the molar mass of the protein. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1


Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
Of an unknown protein are dissolved in enough solvent to make 5.00mL of solution. The osmotic pressu...
Questions

Mathematics, 05.01.2021 08:00

Mathematics, 05.01.2021 08:00



Mathematics, 05.01.2021 08:00

History, 05.01.2021 08:00


Mathematics, 05.01.2021 08:00




Mathematics, 05.01.2021 08:10

English, 05.01.2021 08:10

Mathematics, 05.01.2021 08:10




Social Studies, 05.01.2021 08:10

History, 05.01.2021 08:10

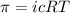
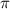 = osmotic pressure of the solution = 0.0604 atm
= osmotic pressure of the solution = 0.0604 atm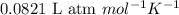
![25^oC=[273+25]=298K](/tpl/images/0538/8757/6a9f9.png)