
Chemistry, 09.03.2020 16:59 BigDough9090
375 mL of a 0.88 M potassium hydroxide solution is added to 496 mL of a 0.76 M cesium hydroxide solution. Calculate the pOH of the resulting solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
375 mL of a 0.88 M potassium hydroxide solution is added to 496 mL of a 0.76 M cesium hydroxide solu...
Questions








Mathematics, 08.04.2021 05:10


Biology, 08.04.2021 05:10

Mathematics, 08.04.2021 05:10

Social Studies, 08.04.2021 05:10

Mathematics, 08.04.2021 05:10

Mathematics, 08.04.2021 05:10


Geography, 08.04.2021 05:10

Mathematics, 08.04.2021 05:10

Mathematics, 08.04.2021 05:10

Health, 08.04.2021 05:10

Mathematics, 08.04.2021 05:10

 in 375 mL of 0.88 M of KOH =
in 375 mL of 0.88 M of KOH =  = 0.33 moles
= 0.33 moles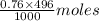 = 0.38 moles
= 0.38 moles![[OH^{-}]](/tpl/images/0538/8908/e46dd.png) =
= 
![pOH=-log[OH^{-}]=-log(0.82)=0.086](/tpl/images/0538/8908/1f1db.png)


