
Chemistry, 09.03.2020 17:02 Kalle91106
Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom.
The four quantum numbers, the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms) have strict rules which govern the possible values.
Identity allowable combinations of quantum numbers for an electron. Select all that apply.
a) n = 3, l = 2, ml = −2, ms = −1/2
b) n = 4, l = 0, ml = 1, ms = −1/2
c) n = 3, l = −2, ml = −2, ms = +1/2
d) n = 5, l = 4, ml = 4, ms = +1/2
e) n = 3, l = 3, ml = 1, ms = +1/2
f) n = 2, l = 1, ml = 0 ,ms = 0

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1


Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
Quantum numbers arise naturally from the mathematics used to describe the possible states of an elec...
Questions

Mathematics, 16.10.2020 14:01



Chemistry, 16.10.2020 14:01

Biology, 16.10.2020 14:01

English, 16.10.2020 14:01

Biology, 16.10.2020 14:01


Mathematics, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01

Biology, 16.10.2020 14:01

Mathematics, 16.10.2020 14:01



Computers and Technology, 16.10.2020 14:01




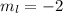 ,
, 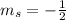
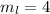 ,
, 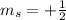
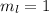 ,
, 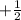 for upward spin and
for upward spin and 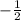 for downward spin.
for downward spin.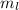 can have values from -l to +l i.e for l= 2 ,
can have values from -l to +l i.e for l= 2 , 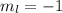 ,
, 


