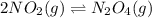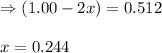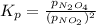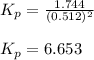Chemistry, 09.03.2020 20:03 dontcareanyonemo
A flask is charged with 1.500 atm of N2O4(g) and 1.00 atm NO2(g) at 25°C, and the following equilibrium is established. 2 NO2(g) ⇌ N2O4(g) At equilibrium, the partial pressure of NO2(g) is 0.512 atm. Calculate the equilibrium constant Kp for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
A flask is charged with 1.500 atm of N2O4(g) and 1.00 atm NO2(g) at 25°C, and the following equilibr...
Questions

Mathematics, 21.04.2021 20:50

History, 21.04.2021 20:50







Mathematics, 21.04.2021 20:50

History, 21.04.2021 20:50




Mathematics, 21.04.2021 20:50

Computers and Technology, 21.04.2021 20:50




Chemistry, 21.04.2021 20:50

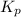 for the given reaction is 6.653
for the given reaction is 6.653