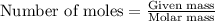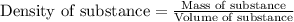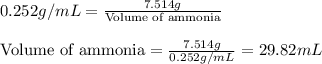Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
Assume that concentrated aqueous NH3 has a density of 0.252 g/mL (0.252 g of NH3 per mL of liquid)....
Questions



Mathematics, 23.04.2020 18:02




World Languages, 23.04.2020 18:02


Computers and Technology, 23.04.2020 18:02

Mathematics, 23.04.2020 18:02


Mathematics, 23.04.2020 18:02


Mathematics, 23.04.2020 18:02


Social Studies, 23.04.2020 18:02



Mathematics, 23.04.2020 18:03