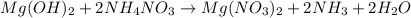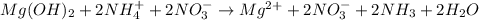Chemistry, 09.03.2020 23:56 SushiMagic
When ammonium nitrate is added to a suspension of magnesium hydroxide in water, the Mg(OH)2 dissolves. Write a net ionic equation to show how this occurs. Do not include physical states and use the smallest possible integer coefficients.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
When ammonium nitrate is added to a suspension of magnesium hydroxide in water, the Mg(OH)2 dissolve...
Questions

Mathematics, 10.05.2021 19:20

Computers and Technology, 10.05.2021 19:20

Mathematics, 10.05.2021 19:20

Mathematics, 10.05.2021 19:20

Mathematics, 10.05.2021 19:20



Mathematics, 10.05.2021 19:20




History, 10.05.2021 19:20


Mathematics, 10.05.2021 19:20

Chemistry, 10.05.2021 19:20


Mathematics, 10.05.2021 19:20

Social Studies, 10.05.2021 19:20

Mathematics, 10.05.2021 19:20

Mathematics, 10.05.2021 19:20

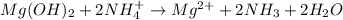
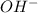 in
in 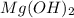 reacts with
reacts with 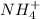 to form
to form 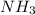 and
and 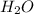 .
.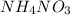 is added to suspension of
is added to suspension of