

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

You know the right answer?
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0025 M...
Questions

History, 17.12.2021 01:30

History, 17.12.2021 01:30

Computers and Technology, 17.12.2021 01:30






English, 17.12.2021 01:30

Computers and Technology, 17.12.2021 01:30









Social Studies, 17.12.2021 01:30

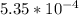 M
M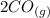 +
+ 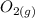 ⇄
⇄ 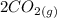
![K_c =\frac{[CO_2]^2}{[CO]^2[O_2]}](/tpl/images/0539/1399/a6d01.png)
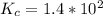 ; Then:
; Then: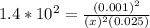
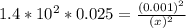
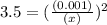
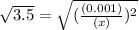
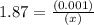
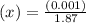
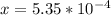 M
M

