
Chemistry, 09.03.2020 23:58 denaeyafranklin8430
Calculate the enthalpy of formation (kJ/mol) of MgO(s). The enthalpy of reaction for the equation as written is 100.8 kJ/mol. If the answer is negative, enter the sign and then the magnitude.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1
You know the right answer?
Calculate the enthalpy of formation (kJ/mol) of MgO(s). The enthalpy of reaction for the equation as...
Questions


Health, 24.02.2021 18:40

Mathematics, 24.02.2021 18:40




Mathematics, 24.02.2021 18:40

Social Studies, 24.02.2021 18:40




Chemistry, 24.02.2021 18:40

Mathematics, 24.02.2021 18:40

Mathematics, 24.02.2021 18:40

Arts, 24.02.2021 18:40

English, 24.02.2021 18:40

Social Studies, 24.02.2021 18:40

Biology, 24.02.2021 18:40

Mathematics, 24.02.2021 18:40

English, 24.02.2021 18:40

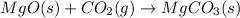
![\Delta H^o_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0539/1394/e893d.png)
![\Delta H_{rxn}=[(1\times \Delta H_f_{(MgCO_3(s))})]-[(1\times \Delta H_f_{(MgO(s))})+(1\times \Delta H_f_{(CO_2(g))})]](/tpl/images/0539/1394/01988.png)
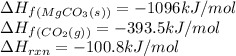
![-100.8=[(1\times (-1096))]-[(1\times \Delta H_f_{(MgO(s))})+(1\times (-393.5))]\\\\\Delta H_f_{(MgO(s))}=-601.7kJ/mol](/tpl/images/0539/1394/d8103.png)


