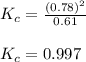Chemistry, 09.03.2020 23:59 pollywallythecat
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium described by the equation N2O4(g) 2NO2(g) If at equilibrium the N2O4 is 39% dissociated, what is the value of the equilibrium constant, Kc, for the reaction under these conditions

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1



Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium descr...
Questions


Mathematics, 20.09.2020 03:01


Advanced Placement (AP), 20.09.2020 03:01




Mathematics, 20.09.2020 03:01





Mathematics, 20.09.2020 03:01

History, 20.09.2020 03:01




Physics, 20.09.2020 03:01

Physics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

 = 0.39
= 0.39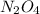 , c =
, c = 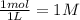

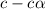
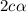
![N_2O_4=c-c\alpha =[1-(1\times 0.39)]=0.61M](/tpl/images/0539/1468/f8a46.png)
![NO_2=2c\alpha =[2\times 1\times 0.39]=0.78M](/tpl/images/0539/1468/5fcc4.png)
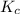 for above equation follows:
for above equation follows:![K_{c}=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0539/1468/1a559.png)