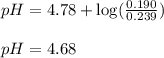Chemistry, 10.03.2020 00:07 laurabwhiddon
Calculate the pH of a solution that is 0.239 M acetic acid and 0.190 M sodium acetate. The Ka of acetic acid is 1.76×10–5 at 25°C. What is the pH of this mixture at 0°C? (At 0°C, Ka = 1.64x10-5)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
Calculate the pH of a solution that is 0.239 M acetic acid and 0.190 M sodium acetate. The Ka of ace...
Questions

History, 01.09.2019 17:20





History, 01.09.2019 17:20


Mathematics, 01.09.2019 17:20

History, 01.09.2019 17:20


Health, 01.09.2019 17:20






Mathematics, 01.09.2019 17:20

Chemistry, 01.09.2019 17:20


![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0539/1827/e4eea.png)
![pH=pK_a+\log(\frac{[CH_3COONa]}{[CH_3COOH]})](/tpl/images/0539/1827/05ea7.png)
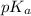 = negative logarithm of acid dissociation constant of acetic acid at 0°C = 4.78
= negative logarithm of acid dissociation constant of acetic acid at 0°C = 4.78![[CH_3COONa]=0.190M](/tpl/images/0539/1827/29d58.png)
![[CH_3COOH]=0.239M](/tpl/images/0539/1827/577dd.png)