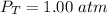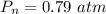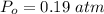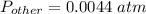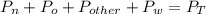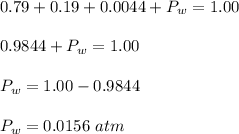Chemistry, 10.03.2020 00:55 ErrorNameTaken505
What is the partial pressure of water vapor in an air sample when the total pressure is 1.00 atm, the partial pressure of nitrogen is 0.79 atm, the partial pressure of oxygen is 0.19 atm, and the partial pressure of all other gases in air is 0.0044 atm?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
What is the partial pressure of water vapor in an air sample when the total pressure is 1.00 atm, th...
Questions

Spanish, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00



Mathematics, 13.01.2021 02:00

English, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00


Mathematics, 13.01.2021 02:00


English, 13.01.2021 02:00


Mathematics, 13.01.2021 02:00

English, 13.01.2021 02:00


Mathematics, 13.01.2021 02:00

Mathematics, 13.01.2021 02:00


Mathematics, 13.01.2021 02:00

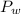 .
.