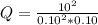Chemistry, 10.03.2020 00:52 jhonnysoriano9053
At 700 K, the reaction 2SO2(g) + O2(g) <> 2SO3(g) has the equilibrium constant Kc = 4.3 x 106. At a certain instant, following concentrations are present in the system: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
At 700 K, the reaction 2SO2(g) + O2(g) <> 2SO3(g) has the equilibrium constant Kc = 4.3 x 106....
Questions

Health, 24.05.2020 02:01



Mathematics, 24.05.2020 02:01



Mathematics, 24.05.2020 02:01



Mathematics, 24.05.2020 02:01






History, 24.05.2020 02:01

History, 24.05.2020 02:01



![Q=\frac{[C]^{c} *[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0539/4103/8be7b.png)
![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0539/4103/e45ae.png)
![Q=\frac{[So_{3}] ^{2} }{[SO_{2} ]^{2}* [O_{2}] }](/tpl/images/0539/4103/dbc4a.png)