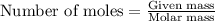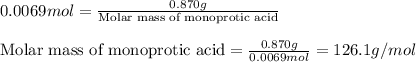Chemistry, 10.03.2020 00:50 kylediedrich1343
A 0.870 g sample of a monoprotic acid is dissolved in water and titrated with 0.300 M KOH.
What is the molar mass of the acid if 23.0 mL of the KOH solution is required to neutralize the sample?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
A 0.870 g sample of a monoprotic acid is dissolved in water and titrated with 0.300 M KOH.
Wha...
Wha...
Questions





Mathematics, 04.12.2020 03:40


Mathematics, 04.12.2020 03:40


Mathematics, 04.12.2020 03:40

English, 04.12.2020 03:40

Mathematics, 04.12.2020 03:40

Mathematics, 04.12.2020 03:40



Mathematics, 04.12.2020 03:40


Mathematics, 04.12.2020 03:40


Mathematics, 04.12.2020 03:40

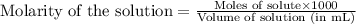

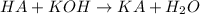
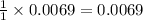 moles of HA
moles of HA