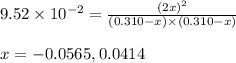He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) + CCl4(g)→ 2CH2Cl2(g)
Calculate the equilibrium concentrations of reactants and product when 0.310 moles of CH4 and 0.310 moles of CCl4 are introduced into a 1.00 L vessel at 350 K.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) +...
CH4(g) +...
Questions

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30



Mathematics, 12.12.2020 16:30

Chemistry, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30




Mathematics, 12.12.2020 16:30






History, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30

Geography, 12.12.2020 16:30

 are 0.2686 M, 0.2686 M and 0.0828 M respectively.
are 0.2686 M, 0.2686 M and 0.0828 M respectively.
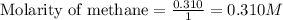


 for above equation follows:
for above equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0539/2756/bf52a.png)