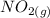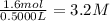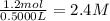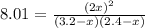Chemistry, 10.03.2020 00:27 TabbyKun00
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becomes possible: +NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.01 at the temperature of the flask. Calculate the equilibrium molarity of NO3 . Round your answer to two decimal places.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3

Chemistry, 23.06.2019 15:30
Acontainer holds 6.4 moles of gas. hydrogen gas makes up 25% of the total moles in the container. if the total pressure is 1.24atm. what is the partial pressure of hydrogen
Answers: 3
You know the right answer?
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becom...
Questions


English, 03.09.2021 08:50

Mathematics, 03.09.2021 08:50



History, 03.09.2021 08:50





Physics, 03.09.2021 08:50




Mathematics, 03.09.2021 08:50


English, 03.09.2021 08:50

Mathematics, 03.09.2021 08:50

Chemistry, 03.09.2021 08:50

Mathematics, 03.09.2021 09:00

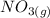 is 1.60 M
is 1.60 M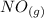 ⇄2
⇄2