Chemistry, 10.03.2020 00:37 serenityarts123
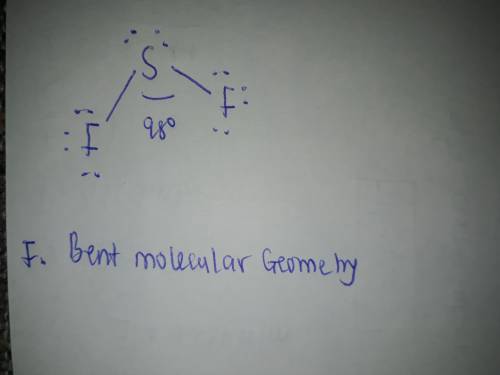
Draw the Lewis structure of SF2, showing all lone pairs. Identify the molecular geometry of SF2.
A. square pyramidal
B. trigonal pyramidal
C. trigonal planar
D. linear
E. T‑shaped
F. bent
G. square planar
H. octahedral
I. trigonal bipyramidal
J. tetrahedral

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

You know the right answer?
Draw the Lewis structure of SF2, showing all lone pairs. Identify the molecular geometry of SF2.
Questions


Mathematics, 05.10.2021 06:20

Chemistry, 05.10.2021 06:20

Chemistry, 05.10.2021 06:20


Chemistry, 05.10.2021 06:20

Mathematics, 05.10.2021 06:20

Mathematics, 05.10.2021 06:20



Mathematics, 05.10.2021 06:20

Biology, 05.10.2021 06:20





Mathematics, 05.10.2021 06:20

Health, 05.10.2021 06:20