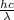Chemistry, 10.03.2020 01:34 tsmalls70988
The first ionization energy, E , of a nitrogen atom is 2.32 aJ. What is the wavelength of light, in nanometers, that is just sufficient to ionize a nitrogen atom? Values for constants can be found in the Chempendix.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
The first ionization energy, E , of a nitrogen atom is 2.32 aJ. What is the wavelength of light, in...
Questions

Spanish, 29.12.2019 17:31

Mathematics, 29.12.2019 17:31


Spanish, 29.12.2019 17:31






Mathematics, 29.12.2019 17:31

Chemistry, 29.12.2019 17:31

Mathematics, 29.12.2019 17:31

Mathematics, 29.12.2019 17:31


Mathematics, 29.12.2019 17:31

Social Studies, 29.12.2019 17:31

Mathematics, 29.12.2019 17:31

Mathematics, 29.12.2019 17:31

Biology, 29.12.2019 17:31

Mathematics, 29.12.2019 17:31