Chemistry, 10.03.2020 01:44 tobyhollingsworth178
In a coffee-cup calorimeter, 1 mol NaOH and 1 mol HBr initially at 28 oC (Celsius) are mixed in 100g of water to yield the following reaction: NaOH + HBr → Na+(aq) + Br-(aq) + H2O(l) After mixing the temperature rises to 88.5 oC. Calculate the change in enthalpy of this reaction. Specific heat of the solution = 4.184 J/(g oC) State your answer in kJ with 3 significant figures. Don't forget to enter the unit behind the numerical answer. The molecular weight of NaOH is 40.0 g/mol, and the molecular weight of HBr is 80.9 g/mol. ΔH =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2
You know the right answer?
In a coffee-cup calorimeter, 1 mol NaOH and 1 mol HBr initially at 28 oC (Celsius) are mixed in 100g...
Questions




Mathematics, 20.10.2020 21:01



Mathematics, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01


History, 20.10.2020 21:01


English, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01

Physics, 20.10.2020 21:01

Chemistry, 20.10.2020 21:01



Chemistry, 20.10.2020 21:01

Social Studies, 20.10.2020 21:01

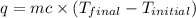
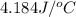
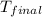 = final temperature =
= final temperature = 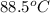
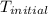 = initial temperature =
= initial temperature = 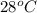
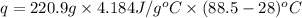
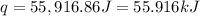 ( J = 0.001 kJ)
( J = 0.001 kJ)
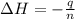
 = enthalpy change = ?
= enthalpy change = ?