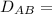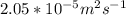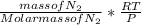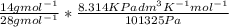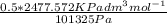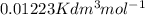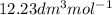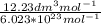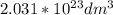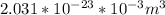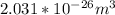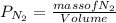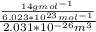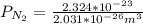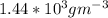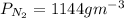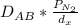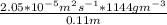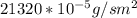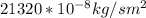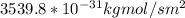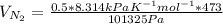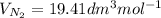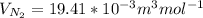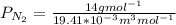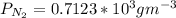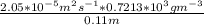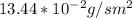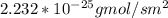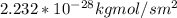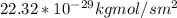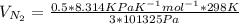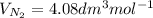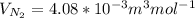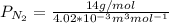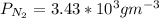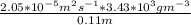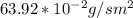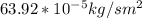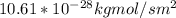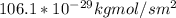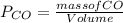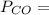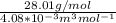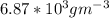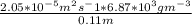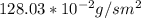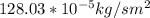Chemistry, 10.03.2020 03:11 devinmoore8686
Equimolar counter-diffusion is occurring at steady state in a tube 0.11 m long containing N2 and CO gases at a total pressure of 1.0 atm absolute. The partial pressure of N2 is 80 mm Hg at one end and 10 mm Hg at the other end. D_AB = 2.05 times 10^5 m^2/s.
a. Calculate the flux in kg mol/s. m^2 at 298 K for N2.
b. Repeat part a, at 473 K. Does the flux increase?
c. Repeat part a, at 298 K, but for a total pressure of 3.0 atm abs. The partial pressures of N2 remain 80 and 10 mm Hg. Does the flux change?
d. Calculate the CO flux for part c.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1

You know the right answer?
Equimolar counter-diffusion is occurring at steady state in a tube 0.11 m long containing N2 and CO...
Questions



Mathematics, 29.03.2021 06:30





Geography, 29.03.2021 06:30



Chemistry, 29.03.2021 06:30

History, 29.03.2021 06:40




Mathematics, 29.03.2021 06:40

Mathematics, 29.03.2021 06:40

Mathematics, 29.03.2021 06:40

Social Studies, 29.03.2021 06:40

Biology, 29.03.2021 06:40

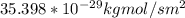
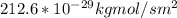
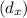 = 0.11 m
= 0.11 m