
Practice Problem 1: The following reagents are combined in a test tube: o 2.00 mL of 0.20 M potassium iodide o 1.00 mL of 1% starch o 0.50 mL of 0.20 M ammonium persulfate o 0.50 mL of 0.012 M sodium thiosulfate o 2.00 mL of 0.20 M potassium nitrate o 2.00 mL of 0.20 M ammonium sulfate What is the concentration of iodide in the solution in the test tube

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Practice Problem 1: The following reagents are combined in a test tube: o 2.00 mL of 0.20 M potassiu...
Questions







Mathematics, 28.05.2020 20:00



Mathematics, 28.05.2020 20:00



Chemistry, 28.05.2020 20:00

Mathematics, 28.05.2020 20:00

History, 28.05.2020 20:01

Geography, 28.05.2020 20:01

Mathematics, 28.05.2020 20:01



Biology, 28.05.2020 20:01

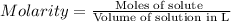
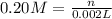
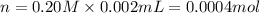
![[KI]=\frac{0.0004 mol}{0.008 L}=0.05 M](/tpl/images/0539/6847/fd685.png)
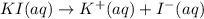
![[I^-]=[KI]=0.05 M](/tpl/images/0539/6847/8ce3a.png)


