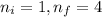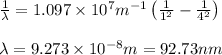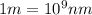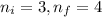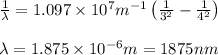Chemistry, 10.03.2020 02:57 urstruulyemily
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible region corresponding to the Balmer series in other series emmission lines are present in different regions of the eletromagnetic spectrum
Calculate the wavelenth of the n=4 to n=1 and the n=4 to n=3 transitions. Indicate in which regions of the electromagnetic spectrum these transitions would occur.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible...
Questions

Mathematics, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00



Mathematics, 04.11.2020 22:00





Mathematics, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00


Mathematics, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00



Mathematics, 04.11.2020 22:00



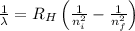
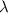 = Wavelength of radiation
= Wavelength of radiation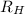 = Rydberg's Constant =
= Rydberg's Constant = 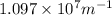
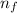 = Higher energy level
= Higher energy level 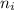 = Lower energy level
= Lower energy level