
Chemistry, 10.03.2020 03:04 ijustneedhelp29
Consider the following equilibrium reaction at a given temperature: A (aq) + 3 B (aq) ⇌ C (aq) + 2 D (aq) Kc = 178. If initially the concentrations of the chemical species are as follows: [A]o = 0.364 M [B]o = 0.170 M [C]o = 0.132 M [D]o = 0.932 M to which direction will the reaction run to reach equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Consider the following equilibrium reaction at a given temperature: A (aq) + 3 B (aq) ⇌ C (aq) + 2 D...
Questions

Mathematics, 19.10.2019 22:30


Health, 19.10.2019 22:30








Mathematics, 19.10.2019 22:30



Biology, 19.10.2019 22:30


Chemistry, 19.10.2019 22:30

History, 19.10.2019 22:30

Social Studies, 19.10.2019 22:30


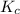 is the constant of a certain reaction at equilibrium while
is the constant of a certain reaction at equilibrium while 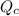 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.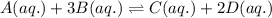
![Q_c=\frac{[D]^2[C]}{[A][B}^3}](/tpl/images/0539/7435/19a81.png)
![[A]_o=0.364M](/tpl/images/0539/7435/6e4ee.png)
![[B]_o=0.170M](/tpl/images/0539/7435/df462.png)
![[C]_o=0.132M](/tpl/images/0539/7435/924d4.png)
![[D]_o=0.932M](/tpl/images/0539/7435/7f66b.png)
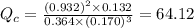

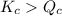 ; the reaction is product favored.When
; the reaction is product favored.When 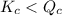 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 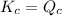 ; the reaction is in equilibrium.
; the reaction is in equilibrium.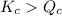 , the reaction will be favoring product side.
, the reaction will be favoring product side.


