
Part A Which of the following statements about chemical formulas is FALSE? Which of the following statements about chemical formulas is FALSE? The subscripts represent the relative mass of each type of atom in the compound. Different compounds made of the same elements have different subscripts. The subscripts represent the relative number of each type of atom in the compound. The subscripts do not change for a given compound. All of the statements are true.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1


Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Part A Which of the following statements about chemical formulas is FALSE? Which of the following st...
Questions

Mathematics, 13.04.2021 23:20

Chemistry, 13.04.2021 23:20

English, 13.04.2021 23:20


History, 13.04.2021 23:20

Arts, 13.04.2021 23:20

Business, 13.04.2021 23:20



Mathematics, 13.04.2021 23:20

Health, 13.04.2021 23:20


Mathematics, 13.04.2021 23:20

Mathematics, 13.04.2021 23:20

Physics, 13.04.2021 23:20

Advanced Placement (AP), 13.04.2021 23:20


Mathematics, 13.04.2021 23:20

Mathematics, 13.04.2021 23:20

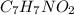 the subscripts present in it are 7, 7, 1, and 2.
the subscripts present in it are 7, 7, 1, and 2.

