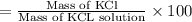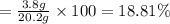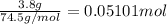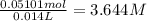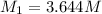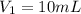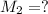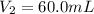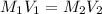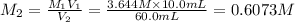Chemistry, 10.03.2020 03:19 jbrown76241
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and KCl solution is 44.30 g. After heating, the evaporating dish and dry KCl have a combined mass of 27.90 g.
(a) What is the mass percent (m/m) of the KCl solution?
(b) What is the molarity ( M) of the KCl solution?
(c) If water is added to 10.0 mL of the initial KCl solution to give a final volume of 60.0 mL, what is the molarity of the diluted KCl solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with...
Questions

Biology, 23.06.2019 17:30


Social Studies, 23.06.2019 17:30



English, 23.06.2019 17:30

History, 23.06.2019 17:30



Mathematics, 23.06.2019 17:30

History, 23.06.2019 17:30


Mathematics, 23.06.2019 17:30



History, 23.06.2019 17:30



Mathematics, 23.06.2019 17:30

Geography, 23.06.2019 17:30