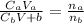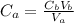Chemistry, 10.03.2020 04:00 dsaefong00
A 23.74-mL volume of 0.0981 M NaOH was used to titrate 25.0 mL of a weak monoprotic acid solution to the stoi- chiometric point. Determine the molar concentration of the weak acid solution. Express your answer to the correct number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
You know the right answer?
A 23.74-mL volume of 0.0981 M NaOH was used to titrate 25.0 mL of a weak monoprotic acid solution to...
Questions

History, 11.07.2019 11:00

Physics, 11.07.2019 11:00

English, 11.07.2019 11:00



Mathematics, 11.07.2019 11:00



Chemistry, 11.07.2019 11:00

Biology, 11.07.2019 11:00


English, 11.07.2019 11:00

Social Studies, 11.07.2019 11:00

Mathematics, 11.07.2019 11:00


Mathematics, 11.07.2019 11:00



Health, 11.07.2019 11:00

English, 11.07.2019 11:00