
Chemistry, 10.03.2020 04:50 MathChic68
The following reaction was performed in a sealed vessel at 791 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.10M and [I2]=2.50M . The equilibrium concentration of I2 is 0.0800 M . What is the equilibrium constant, Kc, for the reaction at this temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
The following reaction was performed in a sealed vessel at 791 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, on...
Questions









English, 07.10.2019 17:10

Mathematics, 07.10.2019 17:10


Biology, 07.10.2019 17:10


English, 07.10.2019 17:10

Social Studies, 07.10.2019 17:10

English, 07.10.2019 17:10

Physics, 07.10.2019 17:10


English, 07.10.2019 17:10

Mathematics, 07.10.2019 17:10

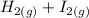 ⇌
⇌ 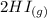
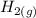

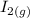 ⇌
⇌ ![K_c = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0540/0427/6b81e.png)
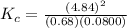
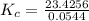
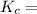 430.62
430.62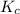 ≅ 431
≅ 431 

