
Chemistry, 10.03.2020 04:49 ngmasuku3115
Be sure to answer all parts. The equilibrium constant, Kc, for the formation of nitrosyl chloride from nitric oxide and chlorine,2NO(g) + Cl2(g) ⇌ 2NOCl(g)is 6.5 ×104 at 35°C. Calculate KP for this reaction, and determine whether the reaction will proceed to the right or to the left to achieve equilibrium when the starting pressures are PNO = 1.01 atm, PCl2 = 0.42 atm, and PNOCl = 1.76 atm.×10(Enter your answer in scientific notation.)reaction will proceed to the rightreaction is at equilibriumreaction will proceed to the left

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Be sure to answer all parts. The equilibrium constant, Kc, for the formation of nitrosyl chloride fr...
Questions


English, 29.08.2019 11:20

Mathematics, 29.08.2019 11:20



History, 29.08.2019 11:20


Chemistry, 29.08.2019 11:20

History, 29.08.2019 11:20

Computers and Technology, 29.08.2019 11:20

History, 29.08.2019 11:20







Health, 29.08.2019 11:20


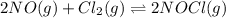
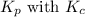 is given by the formula:
is given by the formula: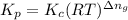
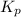 = equilibrium constant in terms of partial pressure = ?
= equilibrium constant in terms of partial pressure = ?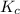 = equilibrium constant in terms of concentration =
= equilibrium constant in terms of concentration = 
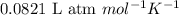
![35^oC=[35+273]K=308K](/tpl/images/0540/0407/bab2f.png)
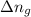 = change in number of moles of gas particles =
= change in number of moles of gas particles = 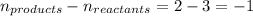
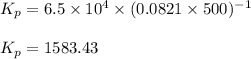
 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.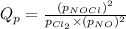
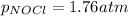
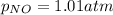
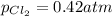
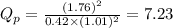
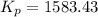
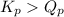 ; the reaction is product favored.When
; the reaction is product favored.When 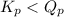 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 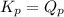 ; the reaction is in equilibrium
; the reaction is in equilibrium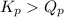 , the reaction will be favoring product side.
, the reaction will be favoring product side.

