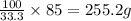Chemistry, 10.03.2020 04:47 morenodonaldo762
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3% w/w solution of acetic acid in ethanol. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3...
Questions

Mathematics, 06.02.2021 01:20

Chemistry, 06.02.2021 01:20


History, 06.02.2021 01:20

Mathematics, 06.02.2021 01:20

Mathematics, 06.02.2021 01:20

Mathematics, 06.02.2021 01:20

English, 06.02.2021 01:20




Mathematics, 06.02.2021 01:20

Mathematics, 06.02.2021 01:20


Mathematics, 06.02.2021 01:20

Spanish, 06.02.2021 01:20

Mathematics, 06.02.2021 01:20

Mathematics, 06.02.2021 01:20


Mathematics, 06.02.2021 01:20