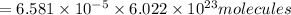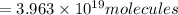Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation
3H2(g)+N2(g)→2NH3(g)
NOTE: Throughout this tutorial use molar masses expressed to five significant figures.
Part A
How many moles of NH3 can be produced from 18.0 mol of H2 and excess N2?
Part B
How many grams of NH3 can be produced from 4.50 mol of N2 and excess H2.
Part C
How many grams of H2 are needed to produce 12.57 g of NH3?
Part D
How many molecules (not moles) of NH3 are produced from 1.99×10−4 g of H2?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation<...
Questions

History, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Chemistry, 24.03.2021 17:40

English, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Biology, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40


History, 24.03.2021 17:40


English, 24.03.2021 17:40


Social Studies, 24.03.2021 17:40

Social Studies, 24.03.2021 17:40

Computers and Technology, 24.03.2021 17:40

English, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

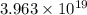 molecules of ammonia will be produced.
molecules of ammonia will be produced.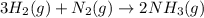
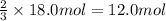 of ammonia
of ammonia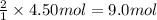 of ammonia
of ammonia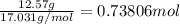
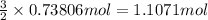 of hydrogen gas
of hydrogen gas 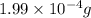
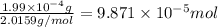
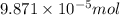 moles of hydrogen gas will give ;
moles of hydrogen gas will give ;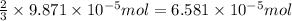 of ammonia.
of ammonia.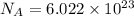 molecules/ atoms
molecules/ atoms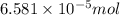 .
.