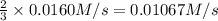Chemistry, 10.03.2020 04:41 flynwildozfuf5
Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of −0.0160 M/s. At what rate is ammonia being formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction...
Questions




English, 27.03.2020 07:21

Mathematics, 27.03.2020 07:22


English, 27.03.2020 07:25

Mathematics, 27.03.2020 07:25



Mathematics, 27.03.2020 07:32

Mathematics, 27.03.2020 07:32




Mathematics, 27.03.2020 07:32


World Languages, 27.03.2020 07:32


Social Studies, 27.03.2020 07:32

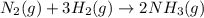
![R=\frac{-1}{1}\frac{d[N_2]}{dt}=\frac{-1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/a62ba.png)
![-\frac{d[H_2]}{dt}=0.0160M/s](/tpl/images/0539/9987/d5dae.png)
![-\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/527e4.png)
![\frac{-1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/2a2d1.png)
![\frac{-1}{3}\frac{d[H_2]}{dt}\times 2=\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/92027.png)
![\frac{1}{3}(-\frac{d[H_2]}{dt})\times 2=\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/e9e1c.png)