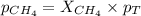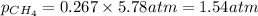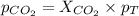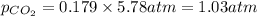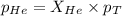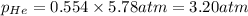Chemistry, 10.03.2020 04:42 erikap0889
Determine the mole fractions and partial pressures of CO2, CH4, and He in a sample of gas that contains 1.20 moles of CO2, 1.79 moles of CH4, and 3.71 moles of He, and in which the total pressure is 5.78 atm.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1


Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
Determine the mole fractions and partial pressures of CO2, CH4, and He in a sample of gas that conta...
Questions

Social Studies, 25.01.2020 02:31


Biology, 25.01.2020 02:31

Biology, 25.01.2020 02:31

History, 25.01.2020 02:31

Social Studies, 25.01.2020 02:31

Computers and Technology, 25.01.2020 02:31


History, 25.01.2020 02:31


Mathematics, 25.01.2020 02:31


English, 25.01.2020 02:31


English, 25.01.2020 02:31

English, 25.01.2020 02:31

English, 25.01.2020 02:31

History, 25.01.2020 02:31

Mathematics, 25.01.2020 02:31

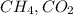 and
and 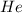 gases are, 0.267, 0.179, 0.554 and 1.54, 1.03 and 3.20 atm respectively.
gases are, 0.267, 0.179, 0.554 and 1.54, 1.03 and 3.20 atm respectively.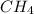 = 1.79 mole
= 1.79 mole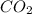 = 1.20 mole
= 1.20 mole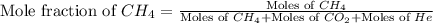
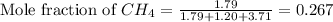
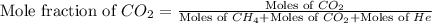
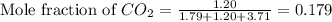
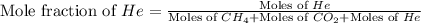
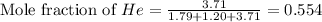
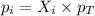
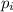 = partial pressure of gas
= partial pressure of gas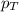 = total pressure of gas = 5.78 atm
= total pressure of gas = 5.78 atm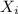 = mole fraction of gas
= mole fraction of gas