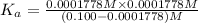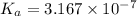Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
A 0.100 mole quantity of a monoprotic acid HA is added to 1.00 L of pure water. When equilibrium is...
Questions

Mathematics, 05.07.2019 20:30

History, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30



Mathematics, 05.07.2019 20:30



English, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30


Mathematics, 05.07.2019 20:30

Mathematics, 05.07.2019 20:30



English, 05.07.2019 20:30



Mathematics, 05.07.2019 20:30

 is the value of
is the value of 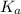 for the acid HA.
for the acid HA.![pH=-log[H^+]](/tpl/images/0540/0009/15713.png)
![3.75=-\log[H^+]](/tpl/images/0540/0009/128bb.png)
![[H^+]=10^{-3.75}=0.0001778 M](/tpl/images/0540/0009/6c87c.png)
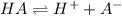
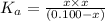
![[H^+]=0.0001778 M](/tpl/images/0540/0009/4f0b0.png)