

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
Rank the following acids in order of increasing acid strength. Key: Weakening of hydrogen bond and s...
Questions

Mathematics, 28.01.2020 09:31


Biology, 28.01.2020 09:31



Social Studies, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31




Mathematics, 28.01.2020 09:31

Biology, 28.01.2020 09:31





Spanish, 28.01.2020 09:31

Physics, 28.01.2020 09:31

Mathematics, 28.01.2020 09:31

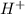 ion.
ion.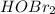 <
< 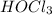 < HOF
< HOF


