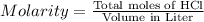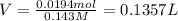Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations would be expected to neutralize. Assume complete neutralization.
a. A tablet containting 350 mg Al(OH)3 and 250 mg Mg(OH)2.
b. A tablet containing 970 mg of CaCO3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 12:20
Describe the structure of ammonium lauryl sulfate. refer to the given diagram. your answer should include the type of bonding, the elements contained, and the size and shape of the molecule. write a short paragraph.
Answers: 3

Chemistry, 23.06.2019 13:30
What is matter? a. anything that has mass and takes up space b. something that has volume and takes up space. c. things that have energy and take up space d. things that take up space but don't have mass
Answers: 2
You know the right answer?
Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations...
Questions

History, 12.04.2021 03:50

History, 12.04.2021 03:50

Mathematics, 12.04.2021 03:50


English, 12.04.2021 03:50




Mathematics, 12.04.2021 03:50



Mathematics, 12.04.2021 03:50


Health, 12.04.2021 03:50



Mathematics, 12.04.2021 03:50

Mathematics, 12.04.2021 03:50


History, 12.04.2021 03:50

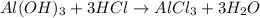
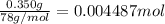
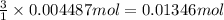 of HCl.
of HCl.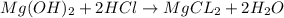
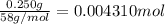
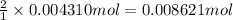 of HCl.
of HCl.
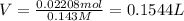
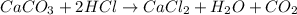
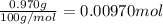
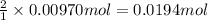 of HCl.
of HCl.