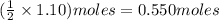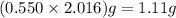Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
Consider the balanced equation. 2HCl + Mg MgCl2 + H2 If 40.0 g of HCl react with an excess of magnes...
Questions


Mathematics, 17.11.2021 01:00

Mathematics, 17.11.2021 01:00



Social Studies, 17.11.2021 01:00

Mathematics, 17.11.2021 01:00





Mathematics, 17.11.2021 01:00

English, 17.11.2021 01:00







Mathematics, 17.11.2021 01:00

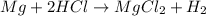
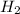 .
.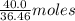 of HCl = 1.10 moles of HCl
of HCl = 1.10 moles of HCl