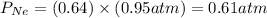Chemistry, 10.03.2020 06:11 jennifer9983
A mixture of He and Ne at a total pressure of 0.95 atm is found to contain 0.32 mol of He and 0.56 mol of Ne. The partial pressure of Ne is atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
A mixture of He and Ne at a total pressure of 0.95 atm is found to contain 0.32 mol of He and 0.56 m...
Questions


Physics, 23.04.2021 17:20

Mathematics, 23.04.2021 17:20


Mathematics, 23.04.2021 17:20




Business, 23.04.2021 17:20



English, 23.04.2021 17:20


Mathematics, 23.04.2021 17:20

Mathematics, 23.04.2021 17:20

Biology, 23.04.2021 17:20




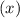 in mixture = (no. of moles of the gas in mixture)/(total no. of moles of gases in mixture)
in mixture = (no. of moles of the gas in mixture)/(total no. of moles of gases in mixture)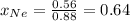
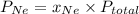
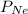 and
and 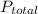 are partial pressure of Ne and total pressure respectively.
are partial pressure of Ne and total pressure respectively.