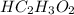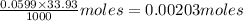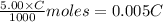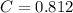Chemistry, 10.03.2020 05:58 ayoismeisalex
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M Ca(OH)2, and 33.93 mL of the Ca(OH)2 solution is required to reach the equivalence point. What is the molarity of the acetic acid? Group of answer choices

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M...
Questions




Physics, 16.09.2019 16:30

Mathematics, 16.09.2019 16:30

Mathematics, 16.09.2019 16:30

Mathematics, 16.09.2019 16:30



Social Studies, 16.09.2019 16:30

Mathematics, 16.09.2019 16:30

Mathematics, 16.09.2019 16:30

Computers and Technology, 16.09.2019 16:30





Health, 16.09.2019 16:30


Mathematics, 16.09.2019 16:30

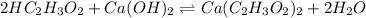
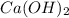 neutralizes 2 moles of
neutralizes 2 moles of