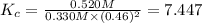Chemistry, 10.03.2020 05:58 emilie3849
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.500 M , [B] = 0.800 M , and [C] = 0.350 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.330 M and [C] = 0.520 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1


Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.500 M , [B] = 0.80...
Questions

Mathematics, 31.07.2019 08:00






Social Studies, 31.07.2019 08:00

Mathematics, 31.07.2019 08:00

Mathematics, 31.07.2019 08:00



History, 31.07.2019 08:00

Mathematics, 31.07.2019 08:10

Mathematics, 31.07.2019 08:10


Biology, 31.07.2019 08:10

History, 31.07.2019 08:10



![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0540/1386/240ef.png)