

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Consider the reaction I2O5(g) 5 CO(g) > 5 CO2(g) I2(g) a) 80.0 grams of iodine(V) oxide, I2O5, re...
Questions




Mathematics, 25.02.2021 20:10

Mathematics, 25.02.2021 20:10


History, 25.02.2021 20:10

Mathematics, 25.02.2021 20:10



Chemistry, 25.02.2021 20:10

Chemistry, 25.02.2021 20:10

History, 25.02.2021 20:10

Social Studies, 25.02.2021 20:10

Chemistry, 25.02.2021 20:10

Social Studies, 25.02.2021 20:10


Mathematics, 25.02.2021 20:10

English, 25.02.2021 20:10

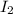 produces=50.8g;
produces=50.8g;
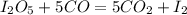
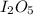 =334g/mole;
=334g/mole; 


