Consider the following reaction, equilibrium concentrations, and equilibrium constant at
a par...

Chemistry, 10.03.2020 06:15 enevjordan
Consider the following reaction, equilibrium concentrations, and equilibrium constant at
a particular temperature. Determine the equilibrium concentration of H2O(g): [C2H4]eq = 0.015
M, [C2H5OH]eq = 1.69 M
C2H4(g) + H2O(g) ? C2H5OH(g) Kc = 9.0

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Questions

Mathematics, 27.04.2021 16:30


History, 27.04.2021 16:30



Advanced Placement (AP), 27.04.2021 16:30

History, 27.04.2021 16:30


Mathematics, 27.04.2021 16:30

Mathematics, 27.04.2021 16:30


Mathematics, 27.04.2021 16:30

Mathematics, 27.04.2021 16:30

Mathematics, 27.04.2021 16:30





Mathematics, 27.04.2021 16:30

Mathematics, 27.04.2021 16:30

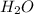 is 12.5 M
is 12.5 M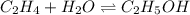
![K_{c}=\frac{[C_{2}H_{5}OH]}{[C_{2}H_{4}][H_{2}O]}](/tpl/images/0540/2224/b68d8.png)
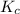 represents equilibrium constant in terms of concentration and species inside third bracket represent equilibrium concentrations
represents equilibrium constant in terms of concentration and species inside third bracket represent equilibrium concentrations![[C_{2}H_{4}]=0.015M](/tpl/images/0540/2224/3b394.png) ,
, ![[C_{2}H_{5}OH]=1.69M](/tpl/images/0540/2224/5d6f4.png) and
and 
![[H_{2}O]=\frac{[C_{2}H_{5}OH]}{[C_{2}H_{4}]\times K_{c}}=\frac{1.69}{0.015\times 9.0}=12.5M](/tpl/images/0540/2224/0a407.png)


