
Chemistry, 10.03.2020 06:31 Halieyrobinson3003
Oxygen is supplied to a medical facility from a 30 ft3 compressed oxygen tank. Initially, the tank is at 2000 psia and 80°F. The oxygen is removed from the tank slowly enough that the temperature in the tank remains at 80°F. After two weeks, the pressure in the tank is 100 psia. Determine the mass of oxygen used in lbm. Also determine the total heat transfer to the tank in Btu. Treat the oxygen as an ideal gas with constant specific heats at 80°F.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2
You know the right answer?
Oxygen is supplied to a medical facility from a 30 ft3 compressed oxygen tank. Initially, the tank i...
Questions

Mathematics, 30.06.2019 19:50

Computers and Technology, 30.06.2019 19:50

Mathematics, 30.06.2019 19:50


English, 30.06.2019 19:50

English, 30.06.2019 19:50

History, 30.06.2019 19:50

Spanish, 30.06.2019 19:50



History, 30.06.2019 19:50

Mathematics, 30.06.2019 19:50

History, 30.06.2019 19:50

History, 30.06.2019 19:50


Mathematics, 30.06.2019 19:50




English, 30.06.2019 19:50

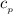 = 0.219 Btu/lbm.R
= 0.219 Btu/lbm.R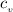 = 0.157 Btu/lbm.R
= 0.157 Btu/lbm.R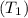 in the tank is given as 80°F.
in the tank is given as 80°F.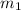 in the tank from the ideal gas equation can be calculated as:
in the tank from the ideal gas equation can be calculated as: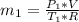
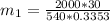

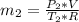
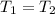 = 540 R
= 540 R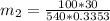
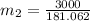
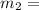 16.57 lbm
16.57 lbm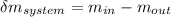
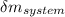 = change in the initial mass and final mass of the oxygen in the tank
= change in the initial mass and final mass of the oxygen in the tank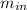 = the inlet mass of the oxygen
= the inlet mass of the oxygen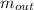 = the outlet mass of the oxygen
= the outlet mass of the oxygen
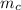 (i.e amount of oxygen used in the system)
(i.e amount of oxygen used in the system)
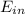 -
- 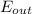 =
= 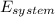
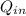 -
-  =
= 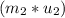 -
- 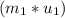
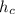 = specific enthalpy of the oxygen used
= specific enthalpy of the oxygen used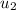 = specific internal energy of the final mass
= specific internal energy of the final mass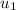 = specific internal energy of the initial mass
= specific internal energy of the initial mass
 &
& 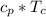
![[m_2*(c_v*T_2)]-[m_1*(c_v*T_1)]+[m_c*(c_p*T_c)]](/tpl/images/0540/2343/fb1ed.png)
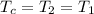
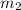 = 16.57 lbm
= 16.57 lbm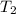 = 540 R
= 540 R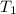 = 540 R
= 540 R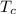 = 540 R
= 540 R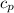 = 0.219 Btu/lbm.R
= 0.219 Btu/lbm.R = 0.157 Btu/lbm.R
= 0.157 Btu/lbm.R = [16.57×(0.157×540)]-[331.38×(0.157×540)]+[314.81×(0.219×540)]
= [16.57×(0.157×540)]-[331.38×(0.157×540)]+[314.81×(0.219×540)]

