

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
NH4+(aq) + NO2−(aq) → N2(g) + 2H2O(l) is given by rate = k[NH4+][NO2−]. At a certain temperature, th...
Questions



Mathematics, 22.09.2021 14:00

Biology, 22.09.2021 14:00

Computers and Technology, 22.09.2021 14:00





Chemistry, 22.09.2021 14:00

Physics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00


Social Studies, 22.09.2021 14:00



History, 22.09.2021 14:00

Biology, 22.09.2021 14:00

English, 22.09.2021 14:00

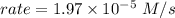
![rate = k[NH_4^+][NO_2^-]](/tpl/images/0540/2238/5a52f.png)
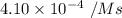
![[NH_4^+]=0.301\ M](/tpl/images/0540/2238/bd4cc.png)
![[NO_2^-]=0.160\ M](/tpl/images/0540/2238/7b7cf.png)
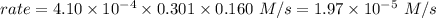
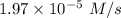 is the rate of the reaction at that temperature if [NH4+] = 0.301 M and [NO2−] = 0.160 M.
is the rate of the reaction at that temperature if [NH4+] = 0.301 M and [NO2−] = 0.160 M.

