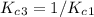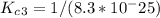Chemistry, 10.03.2020 06:51 Felici6086
Enter your answer in the provided box. The following reactions have the indicated equilibrium constants at a particular temperature:N2(g)+ O2(g)⇌ 2NO(g)Kc = 8.3 ×10−252NO(g)+ O2(g)⇌ 2NO2(g)Kc = 6.4 ×109Determine the value of the equilibrium constant for the following equation at the same temperature:2NO(g)⇌ N2(g)+ O2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1
You know the right answer?
Enter your answer in the provided box. The following reactions have the indicated equilibrium consta...
Questions

Business, 26.10.2021 21:50


English, 26.10.2021 21:50

Mathematics, 26.10.2021 21:50

Mathematics, 26.10.2021 21:50

Physics, 26.10.2021 21:50


Mathematics, 26.10.2021 21:50


Physics, 26.10.2021 21:50

Chemistry, 26.10.2021 21:50

Social Studies, 26.10.2021 21:50





Biology, 26.10.2021 21:50



English, 26.10.2021 21:50

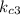 =1.21*
=1.21*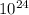
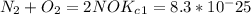 ...................1
...................1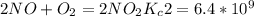 ………..2
………..2
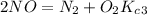 =? ………………………3
=? ………………………3