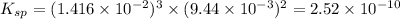Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
1.24 grams of magnesium phosphate tribasic dissolved in 1 L of lemon juice. What is the Ksp of the m...
Questions





History, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

History, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

History, 11.10.2020 14:01



Social Studies, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



Mathematics, 11.10.2020 14:01

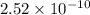
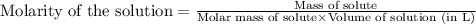
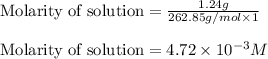
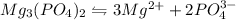
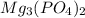 will be:
will be:![K_{sp}=[Mg^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0540/2821/707f8.png)
![[Mg^{2+}]=(3\times 4.72\times 10^{-3})=1.416\times 10^{-2}M](/tpl/images/0540/2821/1537a.png)
![[PO_4^{3-}]=(2\times 4.72\times 10^{-3})=9.44\times 10^{-3}M](/tpl/images/0540/2821/66769.png)