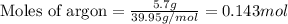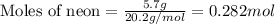Chemistry, 10.03.2020 07:22 lololol270
A gas mixture is made by combining 5.7 g each of Ar , Ne , and an unknown diatomic gas. At STP, the mixture occupies a volume of 12.88 L. What is the molar mass of the unknown gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1


Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
A gas mixture is made by combining 5.7 g each of Ar , Ne , and an unknown diatomic gas. At STP, the...
Questions

Mathematics, 19.04.2021 21:10

History, 19.04.2021 21:10


Mathematics, 19.04.2021 21:10






Mathematics, 19.04.2021 21:10

History, 19.04.2021 21:10



Mathematics, 19.04.2021 21:10

Mathematics, 19.04.2021 21:10

Health, 19.04.2021 21:10




Mathematics, 19.04.2021 21:10

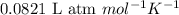
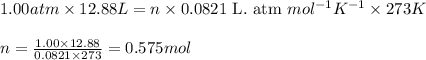
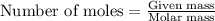 .....(1)
.....(1)