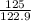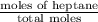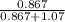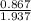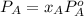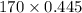Chemistry, 10.03.2020 07:25 starfox5454
At a certain temperature the vapor pressure of pure heptane (C_7 H_16) is measured to be 170. torr. Suppose a solution is prepared by mixing 86.7 g of heptane and 125. g of acetyl bromide (CH_3 COBr). Calculate the partial pressure of heptane vapor above this solution. Round your answer to 3 significant digits. Note for advanced students: you may assume the solution is ideal.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
At a certain temperature the vapor pressure of pure heptane (C_7 H_16) is measured to be 170. torr....
Questions


Business, 25.09.2019 15:00


Geography, 25.09.2019 15:00


Social Studies, 25.09.2019 15:00



Mathematics, 25.09.2019 15:00




Social Studies, 25.09.2019 15:00

Mathematics, 25.09.2019 15:00



Biology, 25.09.2019 15:00



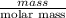
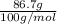
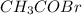 are calculated as follows.
are calculated as follows.