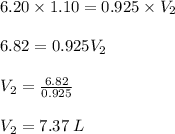Chemistry, 10.03.2020 07:26 laurenppylant
1. A sample of gas has a volume of 6.20 L at 20°C at a pressure of 1.10 atm. What is its volume at the same temperature and at a pressure of 0.925 atm? (Hint: What equation will you use if the temperature is constant?).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

You know the right answer?
1. A sample of gas has a volume of 6.20 L at 20°C at a pressure of 1.10 atm. What is its volume at t...
Questions


Physics, 15.07.2019 23:10


Mathematics, 15.07.2019 23:10
















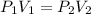
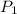 is the original (initial) pressure.
is the original (initial) pressure.
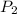 is the final pressure.
is the final pressure.
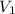 is the original (initial) volume.
is the original (initial) volume.
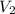 is the final volume.
is the final volume.