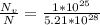Chemistry, 10.03.2020 07:42 gudon986732
For some hypothetical metal, the equilibrium number of vacancies at 600°C is 1 × 1025 m-3. If the density and atomic weight of this metal are 7.40 g/cm3 and 85.5 g/mol, respectively, calculate the fraction of vacancies for this metal at 600°C.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
For some hypothetical metal, the equilibrium number of vacancies at 600°C is 1 × 1025 m-3. If the de...
Questions

Mathematics, 24.03.2021 08:50






Mathematics, 24.03.2021 08:50


Physics, 24.03.2021 08:50

Mathematics, 24.03.2021 08:50






English, 24.03.2021 08:50

Mathematics, 24.03.2021 08:50



Mathematics, 24.03.2021 09:00

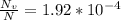
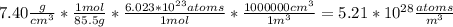
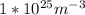 N is the number of atoms per volume calculated above.
N is the number of atoms per volume calculated above.