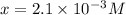Chemistry, 10.03.2020 08:25 ballin2126
The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3×10−5. Calculate the equilibrium concentration of H3O+ in the solution if the initial concentration of C6H5COOH is 7.0×10−2 M .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3×10−5. Calculate the equilibrium co...
Questions



Chemistry, 07.05.2020 04:04


Mathematics, 07.05.2020 04:04

Spanish, 07.05.2020 04:04





Mathematics, 07.05.2020 04:04





Mathematics, 07.05.2020 04:04

Mathematics, 07.05.2020 04:04



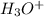 in the solution is,
in the solution is, 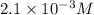
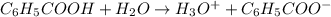
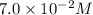
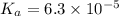
![K_a=\frac{[H_3O^+][C_6H_5COO^-]}{[C_6H_5COOH]}](/tpl/images/0540/6257/de276.png)
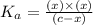
![6.3\times 10^{-5}=\frac{(x)\times (x)}{[(7.0\times 10^{-2})-x]}](/tpl/images/0540/6257/1bf1d.png)